
ABOUT
ScinoPharm Taiwan is a professional pharmaceutical company specializing in high-barrier API and drug product development and manufacturing, dedicated to providing fully integrated solutions as a trusted strategic partner for global clients.
Established in 1997 and headquartered in the Tainan Science Park, ScinoPharm Taiwan operates advanced, FDA- and cGMP-compliant facilities with automated production systems. As an important member of the global pharmaceutical community, ScinoPharm possesses strong R&D and high-standard manufacturing capabilities, maintaining technological leadership and diverse products in the oncology field.
Global Partnerships with Leading Pharmaceutical Companies.
ScinoPharm collaborates with top-tier patented and generic pharmaceutical companies worldwide, including several of the world's top ten. Our expertise spans CNS, cardiovascular, and orphan drug development, supported by an FDA-approved sterile injectable facility that enables a seamless, one-stop solution from API to drug product. With three zero-deficiency FDA inspections, we demonstrate our commitment to world-class quality and regulatory excellence.
In addition to API and drug product development and manufacturing, ScinoPharm offers flexible and efficient CDMO services—from process development and clinical sample preparation to commercial-scale production—tailored to accelerate our partners' innovations to market. Our technology platforms encompass both small molecules and peptides, delivering integrated, reliable, and value-driven solutions to our global partners.



SCINOPHARM
MISSION
Brand Mission
Brand Mission
We apply our expertise with rigorous standards and continuous improvement to deliver safe, effective, and high-quality medicines.
CORE
VALUE
Core Value
Integrity at our core
Scientific expertise
Forward Innovation
Commitment to life



(2001–2025)

MILESTONE
MILESTONE
-
Jul.
Compounding room established and operational
-
Oct.
New brand identity officially established and launched
-
Dec.
Completed third-party limited assurance of GHG emissions (Scopes 1–3) in accordance with the GHG Protocol (2024)
-
Jan.
Carried out ISO 14067 third-party verification of a carbon footprint inventory for one of target products
-
Mar.
The injectable plant passed the third U.S. FDA site inspection
-
Nov.
ScinoPharm Taiwan Achieves Zero-Deficiency Record in ANVISA Inspection
-
Dec.
Completed third-party verification of GHG emissions (Scopes 1–3) in accordance with the GHG Protocol (2023)
-
Sep.
Completed third-party verification of GHG emissions (Scopes 1–2) in accordance with ISO 14064-1
-
May
Passed the eighth U.S. FDA site inspection in Taiwan, and injectable plant passed the first U.S. FDA site inspection
-
May
Adopted the ISO 14064-1 Greenhouse Gas Inventory in 2022 to facilitate planning
-
Nov.
New warehouse building on Taiwan site
-
Dec.
Passed the ninth U.S. FDA site inspection in Taiwan, and injectable plant passed the second U.S. FDA site inspection
-
Apr.
ScinoPharm Injectable Plant received the first GMP inspection by Taiwan Food and Drug Administration(TFDA)
-
Dec.
The new manufacturing facility of the injectable plant at ScinoPharm passed GMP/GDP on-site inspection approved by Taiwan Food and Drug Administration (TFDA)
-
Sep.
SciAnda (Changshu) Pharmaceutical Ltd. had its first China National Medical Products Administration (NMPA) on-site inspection of drug registration and GMP compliance inspection
-
May
Passed the seventh U.S. FDA site inspection in Taiwan
-
Dec.
Developed by ScinoPharm and sold by Baxter, an injectable formulation of adjuvant antiemetic drugs for chemotherapy patients was launched
-
May
SciAnda (Changshu) Pharmaceutical Ltd. passed the first Japanese PMDA site inspections
-
Nov.
The first injectable oncology product was launched in the U.S. market which was the result of our joint venture with SAGENT Pharmaceuticals
-
Feb.
Passed the sixth U.S. FDA site inspection in Taiwan
-
Sep.
Passed the second Japan Pharmaceuticals and Medical Devices Agency (PMDA) site inspection in Taiwan
-
Oct.
Passed the first European Directorate for the Quality of Medicine & HealthCare (EDQM) site inspection in Taiwan
-
Mar.
Passed the fifth U.S. FDA site inspection in Taiwan
-
Oct.
SciAnda (Changshu) Pharmaceutical Ltd. passed U.S. FDA inspection
-
Aug.
SciAnda (Changshu) Pharmaceutical Ltd. passed Mexican healthy authority (COFEPRIS) inspection
-
Jul.
Groundbreaking ceremony for new sterile injectable plant facility, constructed as part of ScinoPharm's vertical integration strategy
-
Aug.
Passed the first European Medicine Agency (EMA) site inspection in Taiwan
-
Nov.
Official inauguration of SciAnda (Changshu) Pharmaceutical Ltd. facility after Phase II construction was completed
-
Aug.
Passed the fourth U.S. FDA plant inspection
-
Nov.
Selected as one of the constituent stocks of the Morgan Stanley Capital International (MSCI) Taiwan Index, the first biotech company in Taiwan elected
-
Dec.
43 DMFs were registered with the FDA in the United States, and a total of 631 DMFs were registered worldwide
-
Sep.
Publicly listed on the Taiwan Stock Exchange Corporation (TWSE), stock code 1789
-
Aug.
Signed an investment cooperation pact with Tanvex Biologics, a US company, and Ruentex Group, to jointly develop Biosimilars
-
Oct.
Listed as an Emerging Stock on the Taipei Exchange, stock code 1789
-
Nov.
Became the first biotechnology pharmaceutical company to be certified as an Authorized Economic Operator (AEO) by the Taiwan Ministry of Finance
-
Aug.
Investment and establishment of SciAnda (Changshu) Pharmaceutical Ltd.
-
Jun.
Passed the plant inspection conducted by Hungarian (NIP), an EU member state
-
Jun.
Passed Japan Pharmaceuticals and Medical Devices Agency (PMDA) plant inspection
-
Sep.
Passed Korean FDA (KFDA) plant inspection
-
Oct.
Passed third U.S. FDA plant inspection
-
Dec.
New and lagger quality control building commenced operations
-
Dec.
Revenue surpassed USD 100 million
-
May
Expansion of production line was completed, including Kilo II and ESP II
-
Oct.
Passed Australian TGA plant inspection
-
Aug.
passed second U.S. FDA plant inspection
-
Nov.
Inauguration of main manufacturing building on Taiwan site
-
Jan.
The first Drug Master File (Drug Master File, DMF) submitted to FDA
-
Feb.
Establishment of the reinvested ScinoPharm (Kunshan) Biotech Ltd.
-
May
Our customer submitted to the abbreviated new drug application (ANDA) for generic drugs to U.S. FDA, being the first one to use the company's active pharmaceutical ingredient (API) Establishment of the reinvested ScinoPharm Biotech Ltd.
-
Jun.
Small manufacturing plant (SMU) begins operations
-
Oct.
Passed first U.S. FDA plant inspection
-
Jan.
Kilo Lab begins operations
-
Mar.
The first batch of Good Manufacturing Practices (Good Manufacturing Practices, GMP) products delivered to clients
-
May
Pilot Plant begins operations
-
Nov.
Mini Plant begins operations
-
Oct.
Relocated into the laboratories and offices in Southern Taiwan Science Park
-
May
The US Food and Drug Administration (FDA) screened the company's plant layout design and validation plan
-
May
Groundbreaking ceremony held at the current site of Southern Taiwan Science Park
-
Nov.
ScinoPharm Taiwan, Ltd. established
















AWARDS
Award Glory
-
Oct.
Awarded the 2025 "Excellence in Corporate Social Responsibility Award" issued by CommonWealth Magazine
-
Sep.
Awarded the 2024 "Excellence in Corporate Social Responsibility Award" issued by CommonWealth Magazine
-
Oct.
Awarded 21st Century Foundation "Net-Zero Industry Competitiveness" Excellence Award
-
Nov.
Awarded Business Weekly 2024 "Top 100 Carbon Competitiveness"
-
Nov.
Awarded the bronze medal of 16th ARTS & BUSINESS AWARDS by Taiwan Ministry of Culture
-
May
Recognized as Best Pharmaceutical R&D & Manufacturing Company – Asia by 《Acquisition International》
-
May
Recognized as Top CMOs IN APAC 2021 by《Pharma Tech Outlook》
-
Dec.
Awarded the bronze medal of 15th ARTS & BUSINESS AWARDS by Taiwan Ministry of Culture
-
Nov.
Received the Authorized Economic Operator Accreditation
-
Dec.
Granted the Excellence in Workplace Health and Safety Award
-
May
Rated top 5% in the 4th "Corporate Governance Evaluation" by Taiwan Stock Exchange Corporation
-
Aug.
Awarded the 2017 "CSR Corporate Citizenship Award" by CommonWealth Magazine
-
Jul.
Awarded the Company of the Year in the 105th import/export performance by Bureau of Foreign Trade, Ministry of Economic Affairs
-
Aug.
Awarded the 2016 "CSR Corporate Citizenship Award" by CommonWealth Magazine
-
Dec.
Awarded second prize in the "Best Investor Relations Service in the Greater China Region" category in the biotechnology industry by IR Magazine, a global investor relations magazine for professionals
-
Jun.
Rated top 5% in the 2nd "Corporate Governance Evaluation" by Taiwan Stock Exchange Corporation
-
Jul.
Awarded the 2016 Taiwan API Manufacturing Company of the Year by Frost & Sullivan
-
Nov.
Awarded the 2016 Excellence in Workplace Health and Safety Award issued by Southern Taiwan Science Park
-
Dec.
Received three Authorized Economic Operators (AEO) certification by Customs Administration, Ministry of Finance
-
Apr.
Rated A++ for two consecutive years in the "Information Disclosure and Transparency" Ranking results by Taiwan Stock Exchange Corporation
-
Jun.
Awarded 4th "National Industrial Innovation Award" by the Ministry of Economic Affairs
-
Jun.
Rated top 5% in the first "Corporate Governance Evaluation" by Taiwan Stock Exchange Corporation
-
Jul.
Awarded the Company of the Year in the 103rd import/export performance by Bureau of Foreign Trade, Ministry of Economic Affairs
-
Aug.
Awarded the "Citizenship Award" for "Top 100 CSR" by CommonWealth Magazine
-
Jun.
Rated A++ in the 11th Information Disclosure and Transparency Ranking results by Taiwan Stock Exchange Corporation
-
Oct.
Ranked as the top Taiwan biotech pharmaceutical corporation in CommonWealth Magazine's Benchmark Enterprise survey for two consecutive years
-
Oct.
Recognized as one of Most Honored Companies in Asia by the authoritative financial magazine "Institutional Investor"; the only Taiwanese biotech company to receive such an award
-
Jan.
Ranked as one of 79 A+ corporations in Taiwan by Global Views Monthly
-
Sep.
Awarded "102nd National Invention and Creation Award" by the Intellectual Property Office, Taiwan Ministry of Economic Affairs
-
Oct.
Ranked as the top Taiwan biotech pharmaceutical corporation in CommonWealth Magazine's Benchmark Enterprise survey
-
Dec.
Recognized as an Authorized Economic Operator (AEO) by the Taiwan Ministry of Finance
-
Jul.
Awarded "Outstanding Bio Industry Golden Award" by the Bio Taiwan Industry Organization
-
Aug.
Ranked as the number two Taiwan biotech pharmaceutical corporation in CommonWealth Magazine's Benchmark Enterprise survey
-
Nov.
Received management systems accreditation by the Taiwan Responsible Care Association (TRCA) in six categories for the second time, including process safety, employee safety, product safety, and emergency response
-
Nov.
Received "Top 100 Innovation Award" by the Industrial Development Bureau, Taiwan Ministry of Economic Affairs
-
Aug.
Received a 5-year "Taiwan Occupational Safety and Hygiene Management System" (TOSHMS) performance accreditation from the Council of Labor Affairs
-
Sep.
Awarded "Industry Contribution Award" by the Taiwan Chemical Industry Association
-
Sep.
Recognized as an Authorized Economic Operator (AEO) by the Taiwan Ministry of Finance
-
Sep.
Became first API plant in Asia to receive "High-Potency Synthetic Pharmaceutical Safety certificate" from SafeBridge, an international biotechnology and pharmaceutical occupational safety and hygiene accreditation company
-
Nov.
Awarded "Outstanding Enterprise Innovation Award" at the Ministry of Economic Affairs’ 17th Annual Industrial Technology Advancement Award ceremony
-
Nov.
Received management systems accreditation by the "Taiwan Responsible Care Association" (TRCA) in six categories, including process safety, employee safety, product safety, and emergency response
-
Dec.
Awarded "Drug Research and Development Science and Technology Golden Award" by the Taiwan Executive Yuan's Department of Health for six products including Docetaxel, Galantamine, and Irinotecan
-
Sep.
Awarded "Outstanding Contribution to Industrial Safety and Hygiene Award" by the Southern Taiwan Science Park Administration

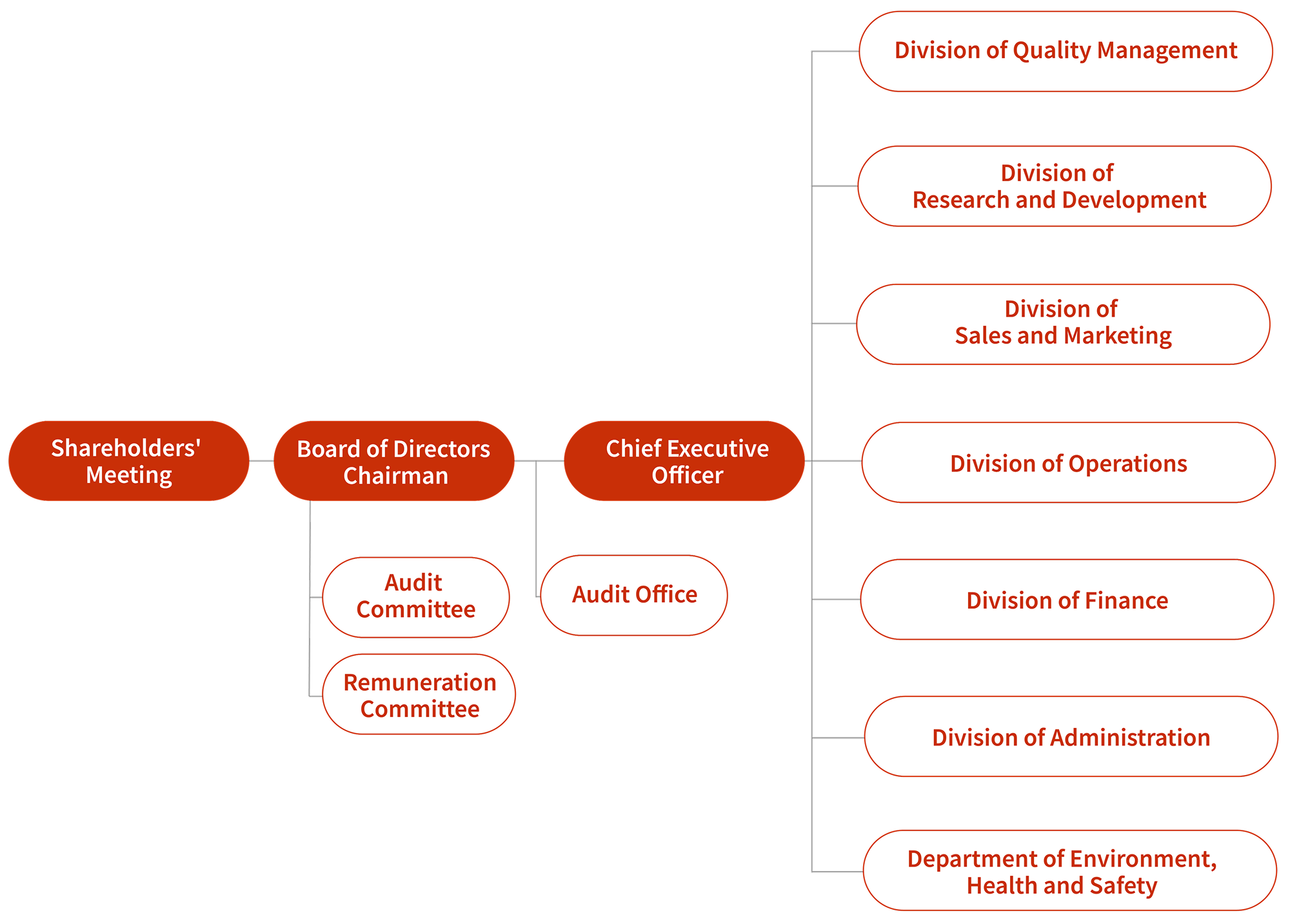
ORGANIZATION
Organization

Chih-Hsien Lo
Chairman and General Chief Strategy Officer- Experience Chairman, Uni-President Enterprises Corp.
- Education MBA from the University of California, Los Angeles, USA

Li-An(Susan) Lu
President and CEO- Experience Over 20 years of experience in financial operations and corporate investment management at Uni-President Enterprises Corp.
-
Education
M.S. in Finance, National Sun Yat-sen University
B.S. in Agricultural Economics, National Taiwan University

LH Lien
Vice-President of Marketing & Sales and Operations- Experience Over 20 years of comprehensive experience in R&D, production, and sales within the pharmaceutical industry
- Education M.S. in Chemical Engineering from National Cheng Kung University

Gloria Chang
Vice President of R&D Division and Chief Scientific Officer- Experience Over 20 years of experience in the pharmaceutical industry Held positions in Quality Control, Analytical R&D, Biotech, Peptide Product Development, Pharmaceutical Development, and Head of Quality Management Division
- Education Ph.D. degree in Chemistry from National Taiwan University

Katy Cheng
Vice President of Quality Management- Experience Over 30 years of experience in drug product quality and production management
-
Education
M.S. in Business Administration, National Chiao-Tung University
B.S. in Pharmacy, Kaohsiung Medical University


__25J14ZqbhK.jpg)