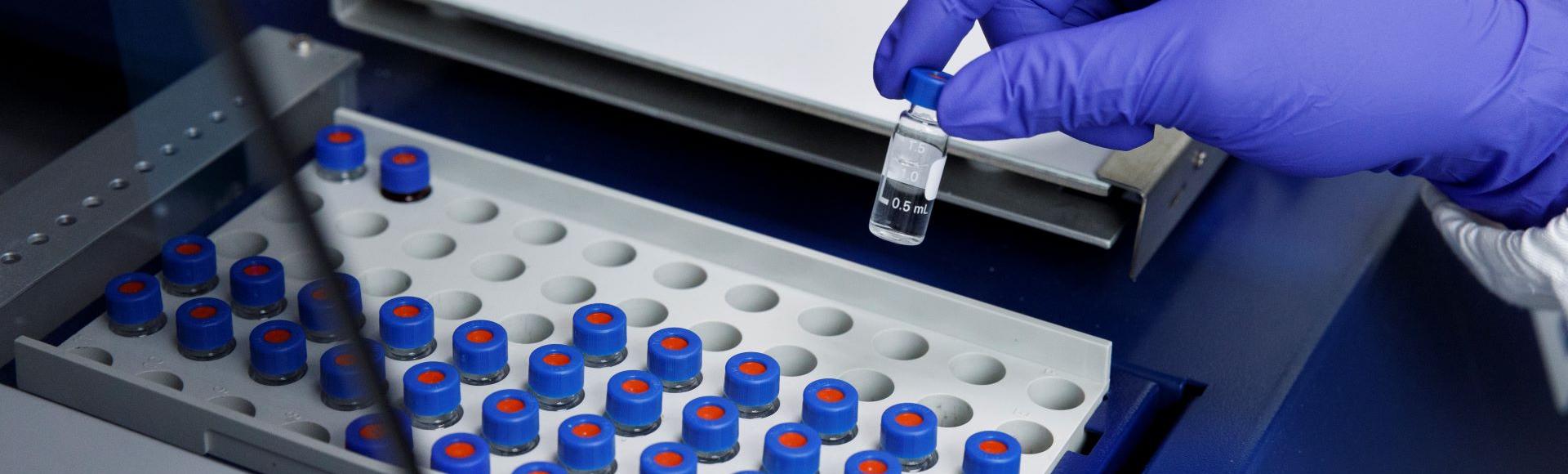
Sterile Injectables Contract Manufacturing
Scinopharm Taiwan offers sterile injectable contract manufacturing services. The production line is designed with high-standard risk management using single-use, disposable filtration-filling components, ensuring strict compliance with the manufacturing standards required for oncology drugs, high-potency drugs, and biologics.
Provide comprehensive lifecycle services, including formulation development, process development, clinical trial-scale manufacturing through to commercial-scale production, analytical method development and validation, as well as commercial packaging and serialization labeling.
Provide comprehensive lifecycle services, including formulation development, process development, clinical trial-scale manufacturing through to commercial-scale production, analytical method development and validation, as well as commercial packaging and serialization labeling.
| Service Scope |
|---|
| Sterile liquid injectables |
| Lyophilized injectables |
| Pre-filled syringes |
| Cartridges |
| Injection pen assembly systems |
| Serialization and secondary packaging |